CX3CL1
| CX3CL1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | CX3CL1, ABCD-3, C3Xkine, CXC3, CXC3C, NTN, NTT, SCYD1, fractalkine, neurotactin, C-X3-C motif chemokine ligand 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 601880; MGI: 1097153; HomoloGene: 2251; GeneCards: CX3CL1; OMA:CX3CL1 - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Fractalkine, also known as chemokine (C-X3-C motif) ligand 1, is a protein that in humans is encoded by the CX3CL1 gene.
Function
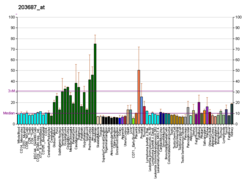
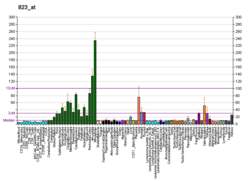
Fractalkine is a large cytokine protein of 373 amino acids, it contains multiple domains and is the only known member of the CX3C chemokine family. It is also commonly known under the names fractalkine (in humans) and neurotactin (in mice).[5][6] The polypeptide structure of CX3CL1 differs from the typical structure of other chemokines. For example, the spacing of the characteristic N-terminal cysteines differs; there are three amino acids separating the initial pair of cysteines in CX3CL1, with none in CC chemokines and only one intervening amino acid in CXC chemokines. CX3CL1 is produced as a long protein (with 373-amino acid in humans) with an extended mucin-like stalk and a chemokine domain on top. The mucin-like stalk permits it to bind to the surface of certain cells. However a soluble (90 kD) version of this chemokine has also been observed. Soluble CX3CL1 potently chemoattracts T cells and monocytes, while the cell-bound chemokine promotes strong adhesion of leukocytes to activated endothelial cells, where it is primarily expressed.[6] CX3CL1 elicits its adhesive and migratory functions by interacting with the chemokine receptor CX3CR1.[7] Its gene is located on human chromosome 16 along with some CC chemokines known as CCL17 and CCL22.[6][8]
Fractalkine is found commonly throughout the brain, particularly in neural cells, and its receptor is known to be present on microglial cells. It has also been found to be essential for microglial cell migration.[9] CX3CL1 is also up-regulated in the hippocampus during a brief temporal window following spatial learning, the purpose of which may be to regulate glutamate-mediated neurotransmission tone. This indicates a possible role for the chemokine in the protective plasticity process of synaptic scaling.[10]
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000006210 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000031778 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Pan Y, Lloyd C, Zhou H, Dolich S, Deeds J, Gonzalo JA, Vath J, Gosselin M, Ma J, Dussault B, Woolf E, Alperin G, Culpepper J, Gutierrez-Ramos JC, Gearing D (1997). "Neurotactin, a membrane-anchored chemokine upregulated in brain inflammation". Nature. 387 (6633): 611–617. Bibcode:1997Natur.387..611P. doi:10.1038/42491. PMID 9177350. S2CID 4307876.
- ^ a b c Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D, Greaves DR, Zlotnik A, Schall TJ (1997). "A new class of membrane-bound chemokine with a CX3C motif". Nature. 385 (6617): 640–644. Bibcode:1997Natur.385..640B. doi:10.1038/385640a0. PMID 9024663. S2CID 4322166.
- ^ Imai T, Hieshima K, Haskell C, Baba M, Nagira M, Nishimura M, Kakizaki M, Takagi S, Nomiyama H, Schall TJ, Yoshie O (1997). "Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion". Cell. 91 (4): 521–530. doi:10.1016/S0092-8674(00)80438-9. PMID 9390561. S2CID 17281691.
- ^ Nomiyama H, Imai T, Kusuda J, Miura R, Callen DF, Yoshie O (1998). "Human chemokines fractalkine (SCYD1), MDC (SCYA22) and TARC (SCYA17) are clustered on chromosome 16q13". Cytogenet. Cell Genet. 81 (1): 10–11. doi:10.1159/000015000. PMID 9691168. S2CID 46851784.
- ^ Maciejewski-Lenoir D, Chen S, Feng L, Maki R, Bacon KB (1999-08-01). "Characterization of fractalkine in rat brain cells: migratory and activation signals for CX3CR-1-expressing microglia". Journal of Immunology. 163 (3): 1628–1635. doi:10.4049/jimmunol.163.3.1628. ISSN 0022-1767. PMID 10415068.
- ^ Sheridan GK, Wdowicz A, Pickering M, Watters O, Halley P, O'Sullivan NC, Mooney C, O'Connell DJ, O'Connor JJ, Murphy KJ (2014). "CX3CL1 is up-regulated in the rat hippocampus during memory-associated synaptic plasticity". Front Cell Neurosci. 8: 233. doi:10.3389/fncel.2014.00233. PMC 4130185. PMID 25161610.
External links
- Human CX3CL1 genome location and CX3CL1 gene details page in the UCSC Genome Browser.
Further reading
- Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D, Greaves DR, Zlotnik A, Schall TJ (1997). "A new class of membrane-bound chemokine with a CX3C motif". Nature. 385 (6617): 640–4. Bibcode:1997Natur.385..640B. doi:10.1038/385640a0. PMID 9024663. S2CID 4322166.
- Combadiere C, Salzwedel K, Smith ED, Tiffany HL, Berger EA, Murphy PM (1998). "Identification of CX3CR1. A chemotactic receptor for the human CX3C chemokine fractalkine and a fusion coreceptor for HIV-1". J. Biol. Chem. 273 (37): 23799–804. doi:10.1074/jbc.273.37.23799. PMID 9726990.
- Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ (1998). "Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity". Proc. Natl. Acad. Sci. U.S.A. 95 (24): 14500–5. Bibcode:1998PNAS...9514500M. doi:10.1073/pnas.95.24.14500. PMC 24402. PMID 9826729.
- Mizoue LS, Bazan JF, Johnson EC, Handel TM (1999). "Solution structure and dynamics of the CX3C chemokine domain of fractalkine and its interaction with an N-terminal fragment of CX3CR1". Biochemistry. 38 (5): 1402–14. doi:10.1021/bi9820614. PMID 9931005.
- Papadopoulos EJ, Sassetti C, Saeki H, Yamada N, Kawamura T, Fitzhugh DJ, Saraf MA, Schall T, Blauvelt A, Rosen SD, Hwang ST (1999). "Fractalkine, a CX3C chemokine, is expressed by dendritic cells and is up-regulated upon dendritic cell maturation". Eur. J. Immunol. 29 (8): 2551–9. doi:10.1002/(SICI)1521-4141(199908)29:08<2551::AID-IMMU2551>3.0.CO;2-T. PMID 10458770.
- Loftus BJ, Kim UJ, Sneddon VP, Kalush F, Brandon R, Fuhrmann J, Mason T, Crosby ML, Barnstead M, Cronin L, Deslattes Mays A, Cao Y, Xu RX, Kang HL, Mitchell S, Eichler EE, Harris PC, Venter JC, Adams MD (1999). "Genome duplications and other features in 12 Mb of DNA sequence from human chromosome 16p and 16q". Genomics. 60 (3): 295–308. doi:10.1006/geno.1999.5927. PMID 10493829.
- Tong N, Perry SW, Zhang Q, James HJ, Guo H, Brooks A, Bal H, Kinnear SA, Fine S, Epstein LG, Dairaghi D, Schall TJ, Gendelman HE, Dewhurst S, Sharer LR, Gelbard HA (2000). "Neuronal fractalkine expression in HIV-1 encephalitis: roles for macrophage recruitment and neuroprotection in the central nervous system". J. Immunol. 164 (3): 1333–9. doi:10.4049/jimmunol.164.3.1333. PMID 10640747. S2CID 20616076.
- Faure S, Meyer L, Costagliola D, Vaneensberghe C, Genin E, Autran B, Delfraissy JF, McDermott DH, Murphy PM, Debré P, Théodorou I, Combadière C (2000). "Rapid progression to AIDS in HIV+ individuals with a structural variant of the chemokine receptor CX3CR1". Science. 287 (5461): 2274–7. Bibcode:2000Sci...287.2274F. doi:10.1126/science.287.5461.2274. PMID 10731151.
- Hoover DM, Mizoue LS, Handel TM, Lubkowski J (2000). "The crystal structure of the chemokine domain of fractalkine shows a novel quaternary arrangement". J. Biol. Chem. 275 (30): 23187–93. doi:10.1074/jbc.M002584200. PMID 10770945. S2CID 38835823.
- Meucci O, Fatatis A, Simen AA, Miller RJ (2000). "Expression of CX3CR1 chemokine receptors on neurons and their role in neuronal survival". Proc. Natl. Acad. Sci. U.S.A. 97 (14): 8075–80. Bibcode:2000PNAS...97.8075M. doi:10.1073/pnas.090017497. PMC 16672. PMID 10869418.
- Papadopoulos EJ, Fitzhugh DJ, Tkaczyk C, Gilfillan AM, Sassetti C, Metcalfe DD, Hwang ST (2000). "Mast cells migrate, but do not degranulate, in response to fractalkine, a membrane-bound chemokine expressed constitutively in diverse cells of the skin". Eur. J. Immunol. 30 (8): 2355–61. doi:10.1002/1521-4141(2000)30:8<2355::AID-IMMU2355>3.0.CO;2-#. PMID 10940926. S2CID 196597758.
- Lucas AD, Chadwick N, Warren BF, Jewell DP, Gordon S, Powrie F, Greaves DR (2001). "The transmembrane form of the CX3CL1 chemokine fractalkine is expressed predominantly by epithelial cells in vivo". Am. J. Pathol. 158 (3): 855–66. doi:10.1016/S0002-9440(10)64034-5. PMC 1850344. PMID 11238035.
- Garton KJ, Gough PJ, Blobel CP, Murphy G, Greaves DR, Dempsey PJ, Raines EW (2001). "Tumor necrosis factor-alpha-converting enzyme (ADAM17) mediates the cleavage and shedding of fractalkine (CX3CL1)". J. Biol. Chem. 276 (41): 37993–8001. doi:10.1074/jbc.M106434200. PMID 11495925.
- Umehara H, Bloom ET, Okazaki T, Nagano Y, Yoshie O, Imai T (2004). "Fractalkine in vascular biology: from basic research to clinical disease". Arterioscler. Thromb. Vasc. Biol. 24 (1): 34–40. doi:10.1161/01.ATV.0000095360.62479.1F. PMID 12969992. S2CID 6183022.
- Umehara H, Tanaka M, Sawaki T, Jin ZX, Huang CR, Dong L, Kawanami T, Karasawa H, Masaki Y, Fukushima T, Hirose Y, Okazaki T (2006). "Fractalkine in rheumatoid arthritis and allied conditions". Mod Rheumatol. 16 (3): 124–30. doi:10.1007/s10165-006-0471-9. PMID 16767549. S2CID 10199059.
- v
- t
- e
-
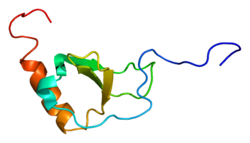
 1b2t: SOLUTION STRUCTURE OF THE CX3C CHEMOKINE DOMAIN OF FRACTALKINE
1b2t: SOLUTION STRUCTURE OF THE CX3C CHEMOKINE DOMAIN OF FRACTALKINE -
 1f2l: CRYSTAL STRUCTURE OF CHEMOKINE DOMAIN OF FRACTALKINE
1f2l: CRYSTAL STRUCTURE OF CHEMOKINE DOMAIN OF FRACTALKINE