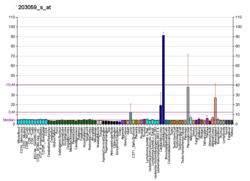
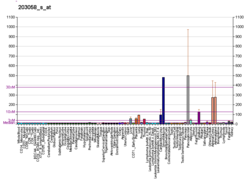
PAPSS2
| PAPSS2 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | PAPSS2, ATPSK2, BCYM4, SK2, 3'-phosphoadenosine 5'-phosphosulfate synthase 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 603005; MGI: 1330223; HomoloGene: 55840; GeneCards: PAPSS2; OMA:PAPSS2 - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Bifunctional 3'-phosphoadenosine 5'-phosphosulfate synthetase 2 is an enzyme that in humans is encoded by the PAPSS2 gene.[5][6]
Sulfation is a common modification of endogenous (lipids, proteins, and carbohydrates) and exogenous (xenobiotics and drugs) compounds. In mammals, the sulfate source is 3'-phosphoadenosine 5'-phosphosulfate (PAPS), created from ATP and inorganic sulfate. Two different tissue isoforms encoded by different genes synthesize PAPS. This gene encodes one of the two PAPS synthetases. Defects in this gene cause the Pakistani type of spondyloepimetaphyseal dysplasia. Two alternatively spliced transcript variants that encode different isoforms have been described for this gene.[6]
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000198682 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000024899 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ ul Haque MF, King LM, Krakow D, Cantor RM, Rusiniak ME, Swank RT, Superti-Furga A, Haque S, Abbas H, Ahmad W, Ahmad M, Cohn DH (Oct 1998). "Mutations in orthologous genes in human spondyloepimetaphyseal dysplasia and the brachymorphic mouse". Nat Genet. 20 (2): 157–62. doi:10.1038/2458. PMID 9771708. S2CID 13108930.
- ^ a b "Entrez Gene: PAPSS2 3'-phosphoadenosine 5'-phosphosulfate synthase 2".
Further reading
- Ewing RM, Chu P, Elisma F, et al. (2007). "Large-scale mapping of human protein-protein interactions by mass spectrometry". Mol. Syst. Biol. 3 (1): 89. doi:10.1038/msb4100134. PMC 1847948. PMID 17353931.
- Gerhard DS, Wagner L, Feingold EA, et al. (2004). "The status, quality, and expansion of the NIH full-length cDNA project: the Mammalian Gene Collection (MGC)". Genome Res. 14 (10B): 2121–7. doi:10.1101/gr.2596504. PMC 528928. PMID 15489334.
- Strausberg RL, Feingold EA, Grouse LH, et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. Bibcode:2002PNAS...9916899M. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932.
- Shimizu C, Fuda H, Lee YC, Strott CA (2002). "Transcriptional regulation of human 3'-phosphoadenosine 5'-phosphosulphate synthase 2". Biochem. J. 363 (Pt 2): 263–71. doi:10.1042/0264-6021:3630263. PMC 1222474. PMID 11931653.
- Fuda H, Shimizu C, Lee YC, et al. (2002). "Characterization and expression of human bifunctional 3'-phosphoadenosine 5'-phosphosulphate synthase isoforms". Biochem. J. 365 (Pt 2): 497–504. doi:10.1042/BJ20020044. PMC 1222679. PMID 11931637.
- Xu ZH, Freimuth RR, Eckloff B, et al. (2002). "Human 3'-phosphoadenosine 5'-phosphosulfate synthetase 2 (PAPSS2) pharmacogenetics: gene resequencing, genetic polymorphisms and functional characterization of variant allozymes". Pharmacogenetics. 12 (1): 11–21. doi:10.1097/00008571-200201000-00003. PMID 11773860.
- Xu Z, Wood TC, Adjei AA, Weinshilboum RM (2001). "Human 3'-phosphoadenosine 5'-phosphosulfate synthetase: radiochemical enzymatic assay, biochemical properties, and hepatic variation". Drug Metab. Dispos. 29 (2): 172–8. PMID 11159808.
- Xu ZH, Otterness DM, Freimuth RR, et al. (2000). "Human 3'-phosphoadenosine 5'-phosphosulfate synthetase 1 (PAPSS1) and PAPSS2: gene cloning, characterization and chromosomal localization". Biochem. Biophys. Res. Commun. 268 (2): 437–44. doi:10.1006/bbrc.2000.2123. PMID 10679223.
- Besset S, Vincourt JB, Amalric F, Girard JP (2000). "Nuclear localization of PAPS synthetase 1: a sulfate activation pathway in the nucleus of eukaryotic cells". FASEB J. 14 (2): 345–54. doi:10.1096/fasebj.14.2.345. PMID 10657990. S2CID 9024784.
- Kurima K, Singh B, Schwartz NB (1999). "Genomic organization of the mouse and human genes encoding the ATP sulfurylase/adenosine 5'-phosphosulfate kinase isoform SK2". J. Biol. Chem. 274 (47): 33306–12. doi:10.1074/jbc.274.47.33306. PMID 10559207.
- Ahmad M, Haque MF, Ahmad W, et al. (1998). "Distinct, autosomal recessive form of spondyloepimetaphyseal dysplasia segregating in an inbred Pakistani kindred". Am. J. Med. Genet. 78 (5): 468–73. doi:10.1002/(SICI)1096-8628(19980806)78:5<468::AID-AJMG13>3.0.CO;2-D. PMID 9714015.
- Kurima K, Warman ML, Krishnan S, et al. (1998). "A member of a family of sulfate-activating enzymes causes murine brachymorphism". Proc. Natl. Acad. Sci. U.S.A. 95 (15): 8681–5. Bibcode:1998PNAS...95.8681K. doi:10.1073/pnas.95.15.8681. PMC 21136. PMID 9671738.
- v
- t
- e
-
 2ax4: Crystal structure of the kinase domain of human 3'-phosphoadenosine 5'-phosphosulphate synthetase 2
2ax4: Crystal structure of the kinase domain of human 3'-phosphoadenosine 5'-phosphosulphate synthetase 2
 | This article on a gene on human chromosome 10 is a stub. You can help Wikipedia by expanding it. |
- v
- t
- e